Families clash as Indian health authorities change COVID guidelines Coronavirus pandemic News

[ad_1]
New Delhi, India – At the end of last month, the Indian government made profound changes to the COVID-19 treatment protocol for hospitals, creating controversy and confusion after throwing out drugs that people knew almost by lettering.
I’ve been reading and reading my family’s prescriptions for months, remembering who to give them to, to quickly send them to local pharmacies. Like thousands of other people, I also read hundreds of requests on social media for these drugs.
So why did he throw out the government? When I searched and searched on Google, I also remembered ivermectin, one of the medications I have now thrown in and studied a lot, that my dermatologist prescribed me in July last year.
“The only pill, tonight,” the doctor said. I was told that it is a drug against parasites, which is used for deworming and debris.
Little did he know that within two months I would be prescribed ivermectin as part of COVID treatment.
In a telephone consultation, the doctor gave him five days of doxycycline (an antibacterial medicine) and one day of ivermectin.
Later that day, when government health workers called us, COVID-19 was negative for my husband and I was given a pack of hydroxychloroquine and told to take the pill for seven days.
We both jumped.
A well-known drug of the time, his demand intensified after former U.S. President Donald Trump threatened to shut down medical supplies if India reduced exports to the United States.
This was still the first wave. While the tragedies of Indian migrants going home were endless, people lost their livelihoods and there was a massive economic slowdown caused by the blockade, the death rate was low and hospitals and oxygen were few and far between.
The second wave, which we all thought would be weaker in most accounts, had not yet destroyed us.
In April, a second wave hit our home. My entire family – my husband, my mother and my brothers-in-law – got COVID-19, although some were vaccinated. We were caught in the middle of a hard-hitting appearance when hospital beds, oxygen and medications were in short supply.
COVID was positive my father-in-law was given ivermectin for a week. He was also given the antiviral drug favipiravir, better known as Fabiflu.
When he arrived at the hospital on May 2 for pulmonary pneumonia, he was already being given ivermectin, doxycycline, azithromycin and favipiravir. At the hospital, remdesivir and plasma therapy were administered twice.
We worked on phone lines to get the hospital bed and oxygen concentrator. We did the same thing with my mother when she was admitted on May 11th.
The only difference was that when he arrived at the hospital for pulmonary pneumonia, the therapy was removed from the government’s health protocol for coronavirus.
But this was in April 2021. The Indian Medical Research Council (ICMR), the country’s leading scientific body, declared the “inappropriate use of plasma therapy” in November 2020.
In a statement released on November 17, 2020, the ICMR said that “PLACID, the world’s largest plasma trial, has not shown any benefit to CPT in patients.”
On June 10 this year, Samiran Panda, head of ICMR’s epidemiology and infectious diseases division, confirmed to me that plasma therapy has been shown to be of no benefit to ICMR.
Among other things, in medications like ivermectin, he said all evidence was based on observational studies, which he said could be helpful, but there was no “randomized, double-blind, randomized, controlled clinical trial for efficacy.”
“There is not strong enough evidence to use doxycycline, favipiravir and hydroxychloroquine,” he said.
Many of these medications were part of the health ministry’s COVID-19 clinical guidelines until May 27th. But it was not approved by the World Health Organization or the U.S. Food and Drug Administration (FDA).
However, many doctors believe that plasma therapy saved lives.
Dr. Manoj Luthra, director of the heart surgery department at Jaypee Hospital in Noida, a city outside New Delhi, told me that “although the government could catch plasma therapy, there is enough anecdotal evidence with doctors about its effectiveness. The method of treating COVID-19.”
“Some of us have even written to the government about it,” he said, sharing an article in the American Journal of Therapeutics published in an article called Emerging Evidence Demonstrating Ivermectin Effectiveness in Prophylaxis and Treatment in COVID-19.
 Article shared by Dr. Manoj Luthra in the American Journal of Therapeutics [Al Jazeera]
Article shared by Dr. Manoj Luthra in the American Journal of Therapeutics [Al Jazeera]
But by April there was widespread concern about other drugs, such as the widespread use of steroids in the treatment of coronaviruses. Both my mother and my father-in-law gave me steroids more than a month.
In May, we learned that the government’s new guidelines would recommend that hospitals use steroids for more than 10 days or until the time of hospitalization, whichever comes first.
COVID-19 is considered a life-saving steroid that has been instrumental in reducing lung inflammation if given to patients at the right time and at the right duration. But they also reduce immunity and increase blood sugar levels.
Bishnu Panigrahi, head of Fortis’s medical strategy team, one of India’s largest private hospital teams, said “in terms of steroids, they have been misused”.
He said the steroids were “used very early, used in higher doses and for longer periods of time and were used as secondary infections”.
“The black fungus is a fallout from the misuse of steroids,” he said.
Black mushroom, or mucormycosis, is a rare infection but life-threatening with a 50 percent mortality rate. It affects the sinuses, brain and lungs and can be severe in severely immunocompromised patients.
In India, the disease has risen by 150%, with 31,216 infections and 2,109 deaths so far in the second wave.
The black fungus has now been listed as an epidemic by the Indian Mushroom Law.
As concerns about overuse of prescription drugs escalated, the government made major changes to its health protocol late last month.
On 27 May, the Directorate General of Health Services (DGHS), under the Ministry of Health, effectively discharged all commonly prescribed medicines from the doctor, including ivermectin, azithromycin, doxycycline, zinc, favipiravir and plasma therapy.
The DGHS recommended only antipyretics (medications used to prevent or reduce fevers) and antitussives (to relieve or eliminate coughs) for mild patients, and not to take medications or research for asymptomatic patients.
Steroids and anticoagulants were recommended for moderate cases, and immediate oxygen therapy, intubation, ventilation, and immunomodulators were added in severe cases.
The medical authorities maintained remdesivir (for moderate and severe cases) and tocilizumab without an immunosuppressor label for severe and severe patients.
Many of the new DGHS guidelines were welcomed by, among others, WHO chief scientist Soumya Swaminathan tweeted: “Guidelines based on DGHS evidence @mohfw – easy, rational and clear guidance for physicians.”
Swaminathan said the guidelines are “available when new evidence is available and can be updated.”
However, guidelines issued by the Ministry of Health and the ICMR continue to recommend ivermectin and hydroxychloroquine for mild to moderate cases. They have not been updated or changed until this report was published.
Currently, COVID-19 guidelines are issued by the DGHS, the federal health ministry, the ICMR, and state medical boards. And they are not always the same.
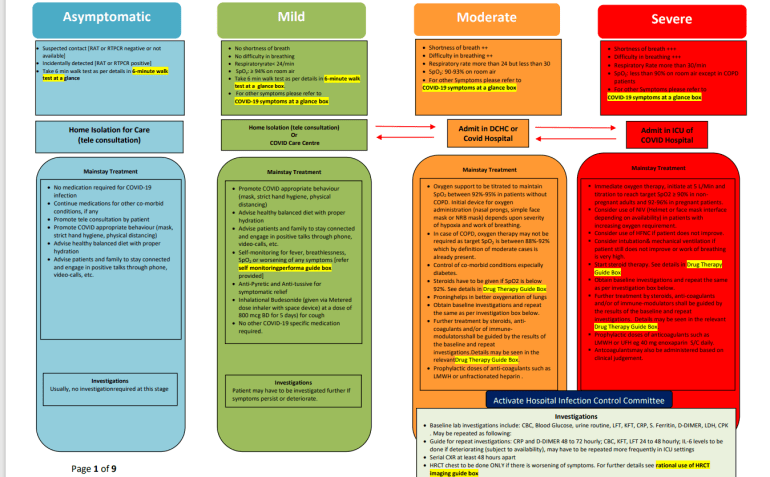 COVID guidelines issued by the Directorate General of Health Services of India (DGHS). [Al Jazeera]
COVID guidelines issued by the Directorate General of Health Services of India (DGHS). [Al Jazeera]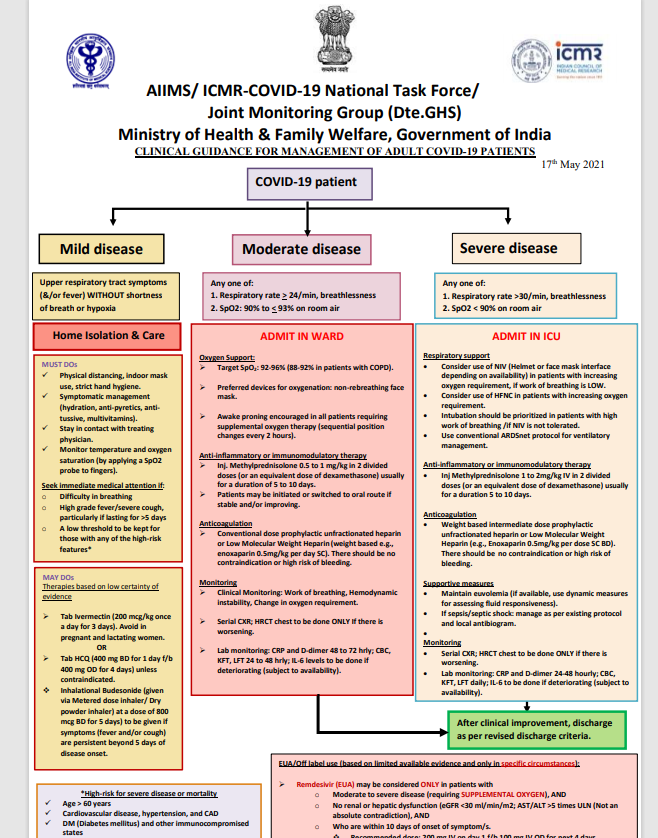 Guidelines provided by the Indian Institute of Medical Science (ICMR). [Al Jazeera]
Guidelines provided by the Indian Institute of Medical Science (ICMR). [Al Jazeera]
Thus, despite the decline in almost all conventional drugs used so far against COVID-19, remdesivir, which is a broad-spectrum anti-virus drug used in the treatment of hepatitis C, has been maintained by the DGHS in its health protocol. The drug has also been approved by the FDA and is used in many other countries to treat coronaviruses.
But like everything else, there is no open consensus on that either.
Panigrahi of Fortis Hospital says that “there has been a slight use of remdesivir, which is due to the fact that the drugs have aggressively promoted this drug, although the literature has not shown a significant reduction in mortality.”
However, DS Rana, superintendent of Sir Delhi’s Ram Ganga Ram Hospital in New Delhi, one of the largest COVID facilities in the capital, says it is an effective anti-virus medication.
“Well-conducted trials have shown that if this drug is used early in the virus phase, it can have a positive effect on early recovery from COVID in terms of hospitalization. The DGHS has recommended its sensible use,” he said.
 ZIU Sir Ganga Ram Hospital in New Delhi [Courtesy of Sir Ganga Ram Hospital]
ZIU Sir Ganga Ram Hospital in New Delhi [Courtesy of Sir Ganga Ram Hospital]
Although there may be an ideal treatment mechanism in the current scenario, and trial and error are included in the fight against an unknown virus, evidence needs to be reviewed repeatedly to reduce the long-term effects of experimental drugs to change treatment decisions.
According to government approval, the third wave of the virus is “inevitable”.
Experts say the precedents and examples set in the US and Europe suggest that the inclusion of at least 30-40% of the population by the end of the year could be an effective deterrent to the virus.
To date, less than five per cent of India’s 950 million adult population has been fully integrated.
The total number of COVID-19s in the country is now nearly 30 million, and as of Thursday, 381,903 deaths had been recorded. About 200,000 deaths occurred in the second COVID wave.
As a result, concerns are growing about re-infections, re-hospitalization and secondary infections, although daily cases have dropped significantly in the last two weeks.
The Indians hope that the new centralized government vaccination policy announced on June 7 will further intensify the case.
Under the new policy in effect from June 21, the federal government will receive 75% of manufactured vaccine doses directly and administer them free of charge. The remaining 25% of the shots will be available in private hospitals.
As global trends show that transmission and death rates are declining as vaccines increase, the Government of India needs to streamline clinical treatment protocols for COVID-19 and align with global trends.
As for my family, my mother is recovering from a secondary infection and my father-in-law continues to have post-KOVID complications.
[ad_2]
Source link
